Note-Students you must paste the syllabus in your notebook
SCIENCE (Code No. 086)
Class: IX and X (2019-20)
The subject of
Science plays an important role in developing well-defined abilities
in
cognitive, affective and psychomotor domains in
children. It augments the spirit of enquiry, creativity, objectivity and aesthetic sensibility.
Upper primary
stage demands that a number of opportunities should be provided to the students
to
engage
them with the
processes
of Science like observing, recording observations, drawing, tabulation, plotting graphs, etc., whereas the secondary
stage also expects abstraction and quantitative reasoning to occupy a more central place in the teaching and learning of Science. Thus, the
idea of atoms and molecules being the building blocks of
matter
makes its appearance, as
does
Newton’s
law of gravitation.
The present syllabus has been
designed
around seven
broad themes viz. Food; Materials;
The World of The Living; How Things Work; Moving Things, People and Ideas; Natural
Phenomenon and Natural Resources. Special care has been
taken to avoid
temptation of adding too many concepts than can be comfortably learnt in the given time frame. No attempt has been made to be comprehensive.
At this stage, while science is still a common subject, the disciplines of Physics, Chemistry
and
Biology begin to emerge. The students should be exposed to
experiences based on
hands on activities as
well as
modes
of
reasoning that are typical of the subject.
General Instructions:
1. There will be an Annual examination based on entire syllabus.
2. The annual examination will be of 80 marks and 20 marks shall be for Internal
Assessment.
3. The components of Internal Assessment would be:
a. Periodic Assessment of 10 marks that would include:
• For 5 marks- Three periodic tests conducted by the school. Average of the
best two tests to be taken. This will have a
weightage
of
05 marks towards
the
final result.
• For 5 marks- Diverse methods of assessment as per the need of the class
dynamics and curriculum transaction. These may include- short tests, oral
test, quiz, concept map, etc. This will also have a
weightage
of
05 marks towards
the
final result.
b. Practical / Laboratory work should be done throughout the year and the student
should
maintain
record of the same. Practical Assessment should be
continuous. There will be weightage of 5
marks towards the final result. All practicals listed in
the syllabus must be completed.
c. Portfolio to be prepared by the student- This would include classwork, other sample of student work, self-assessment and peer-assessment. This will carry a weightage of 5 marks
towards
the
final results.
COURSE STRUCTURE CLASS IX
(Annual Examination) Marks: 80
|
Unit No.
|
Unit
|
Marks
|
Periods
|
|
I
|
Matter - Its Nature and Behaviour
|
23
|
50
|
|
II
|
Organization in the Living World
|
20
|
45
|
|
III
|
Motion, Force and Work
|
27
|
60
|
|
IV
|
Our Environment
|
06
|
15
|
|
V
|
Food; Food Production
|
04
|
10
|
|
|
Total
|
80
|
|
|
|
Internal assessment
|
20
|
|
|
|
Grand Total
|
100
|
|
Theme: Materials
(50 Periods) Unit
I: Matter-Nature and
Behaviour
Definition of matter; solid, liquid and gas; characteristics - shape, volume, density; change of state-melting (absorption of heat), freezing, evaporation (cooling by
evaporation),
condensation, sublimation.
Nature of matter: Elements, compounds and
mixtures. Heterogeneous and homogenous
mixtures, colloids and suspensions.
Particle
nature, basic
units: Atoms and
molecules, Law of constant
proportions, Atomic
and
molecular masses. Mole concept: Relationship of mole to mass of the particles
and
numbers.
Structure of atoms: Electrons, protons and neutrons, valency, chemical formula of
common compounds. Isotopes and Isobars.
Theme: The World of the Living
(45 Periods) Unit
II: Organization in
the Living World
Cell - Basic
Unit of life : Cell as a
basic unit of life; prokaryotic and
eukaryotic cells,
multicellular organisms; cell membrane and
cell wall, cell organelles and cell inclusions; chloroplast, mitochondria, vacuoles, endoplasmic reticulum, Golgi apparatus; nucleus,
chromosomes - basic structure, number.
Tissues, Organs, Organ
System, Organism:
Structure and functions of animal
and plant tissues
(only four types of
tissues in animals; Meristematic and Permanent tissues in plants).
Biological Diversity: Diversity of plants and animals-basic issues in scientific
naming,
basis
of
classification. Hierarchy of categories
/ groups, Major groups of plants
(salient features)
(Bacteria, Thallophyta, Bryophyta, Pteridophyta, Gymnosperms and
Angiosperms). Major groups of animals (salient features) (Non-chordates upto phyla
and chordates upto classes).
Health and Diseases: Health
and
its
failure. Infectious and
Non-infectious diseases,
their causes and manifestation. Diseases caused by microbes (Virus, Bacteria and Protozoans) and their prevention; Principles of treatment and prevention. Pulse Polio
programmes.
Theme: Moving
Things, People and Ideas
(60 Periods) Unit
III: Motion, Force and Work
Motion: Distance and displacement, velocity; uniform and non-uniform motion
along a straight line; acceleration, distance-time and velocity-time graphs for uniform motion and uniformly
accelerated motion, derivation of equations of motion by graphical
method; elementary idea of uniform circular motion.
Force and Newton’s
laws
: Force and Motion, Newton’s Laws of Motion, Action and
Reaction
forces, Inertia
of
a body, Inertia
and
mass, Momentum, Force and Acceleration. Elementary idea of conservation of Momentum.
Gravitation: Gravitation; Universal Law of Gravitation, Force of Gravitation of the earth
(gravity), Acceleration due to Gravity; Mass and Weight; Free fall.
Floatation: Thrust and Pressure. Archimedes’ Principle; Buoyancy; Elementary idea of
Relative Density.
Work, energy and power: Work done by a
Force, Energy, power; Kinetic and Potential
energy; Law of conservation of energy.
Sound: Nature of sound and its propagation in various media, speed of sound, range of
hearing in humans; ultrasound; reflection of sound; echo and SONAR.
Structure of the
Human Ear (Auditory aspect only).
Theme: Natural Resources: Balance in
nature (15 Periods) Unit
IV: Our Environment
Physical resources: Air, Water,
Soil. Air for respiration, for combustion, for
moderating temperatures; movements of air and its role in bringing rains across India.
Air,
water and soil pollution (brief introduction). Holes
in
ozone layer and the probable damages.
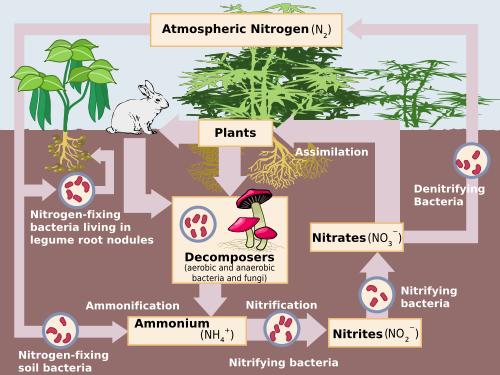
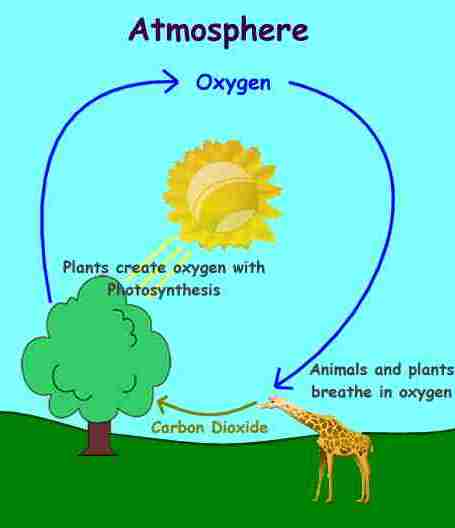
Bio-geo chemical cycles in nature: Water, Oxygen, Carbon and Nitrogen.
Theme: Food
(10 Periods) Unit
V: Food Production
Plant and animal breeding and selection for quality improvement and management; Use
of fertilizers and manures; Protection from pests and diseases; Organic farming.
PRACTICALS
(30 Periods)
Practicals should be
conducted alongside the concepts taught in theory classes. (LIST OF EXPERIMENTS)
1. Preparation of:
a) a true solution of common salt, sugar and alum
b) a suspension of soil, chalk
powder and fine sand in water
c) a
colloidal solution
of
starch in water and egg albumin/milk
in water
and
distinguish between these on the basis
of
transparency
filtration criterion
stability
2. Preparation of
a) A mixture
b) A compound
using iron filings
and sulphur powder
and
distinguishing between these on the basis of:
(i)
appearance, i.e., homogeneity and heterogeneity
(ii)
behaviour
towards a magnet
(iii) behaviour
towards carbon disulphide as a solvent
(iv)
effect of heat
3. Separation of the components of a mixture of sand, common salt and ammonium
chloride (or camphor).
4. Perform the following reactions and classify them as physical or
chemical changes:
a) Iron with copper sulphate solution in water
b) Burning of magnesium ribbon in air
c) Zinc with dilute sulphuric acid
d) Heating of copper
sulphate crystals
e) Sodium sulphate with barium chloride in the form of their solutions
in water
5. Preparation
of stained temporary mounts of (a) onion peel, (b) human cheek
cells
& to record observations and draw their
labeled diagrams.
6. Identification of Parenchyma, collenchyma
and
Sclerenchyma tissues in plants, striped, smooth and cardiac muscle fibers and nerve cells in animals, from
prepared slides.
Draw their
labeled diagrams.
7. Determination of the melting point of ice and the boiling point of water.
8. Verification of the Laws of reflection of sound.
9. Determination
of the density of solid
(denser than water)
by
using a spring balance and a
measuring
cylinder.
10. Establishing the relation between the loss
in
weight of a solid when fully immersed in
a) Tap water
b) Strongly salty
water with
the
weight of water
displaced
by
it by taking at least two
different solids.
11. Determination of the speed of a pulse propagated through a stretched string/slinky(helical spring).
12. Study
of the characteristics of Spirogyra, Agaricus, Moss, Fern, Pinus (either with male
or female
cone) and
an Angiospermic plant. Draw and give two identifying features of the groups
they belong to.
13. Observe
the given pictures/charts/models of earthworm, cockroach, bony fish and
bird.
For each organism,
draw their picture and record:
a) one specific feature of its phylum.
b) one adaptive feature with reference to its habitat.
14. Verification of the law of conservation of mass in a chemical reaction.
15. Study of the external
features of root, stem, leaf and flower
of
monocot and dicot
plants.
COURSE STRUCTURE: CLASS X
(Annual Examination)
Marks: 80
|
Unit
No.
|
Unit
|
Marks
|
Periods
|
|
I
|
Chemical Substances-Nature and Behaviour
|
25
|
55
|
|
II
|
World of Living
|
23
|
50
|
|
III
|
Natural Phenomena
|
12
|
23
|
|
IV
|
Effects of Current
|
13
|
32
|
|
V
|
Natural Resources
|
07
|
20
|
|
|
Total
|
80
|
|
|
|
Internal assessment
|
20
|
|
|
|
Grand
Total
|
100
|
|
Theme : Materials
(55 Periods) Unit
I: Chemical Substances - Nature
and Behaviour
Chemical reactions: Chemical equation, Balanced chemical equation, implications of a
balanced chemical equation,
types
of chemical reactions:
combination, decomposition, displacement, double displacement, precipitation, neutralization, oxidation and reduction.
Acids, bases and salts: Their definitions in terms of furnishing of H+ and OH– ions,
General
properties, examples and uses, concept of pH scale (Definition
relating to
logarithm not required), importance of pH in everyday life; preparation and uses of
Sodium Hydroxide, Bleaching powder, Baking soda, Washing soda and Plaster of Paris.
Metals and nonmetals: Properties of metals and non-metals; Reactivity series; Formation and properties
of
ionic compounds;
Basic
metallurgical processes; Corrosion and its prevention.
Carbon compounds: Covalent
bonding in carbon compounds.
Versatile nature
of carbon. Homologous series. Nomenclature of carbon compounds containing
functional
groups (halogens,
alcohol,
ketones, aldehydes,
alkanes
and
alkynes),
difference
between saturated hydrocarbons and unsaturated hydrocarbons. Chemical properties of carbon compounds (combustion, oxidation, addition and
substitution
reaction). Ethanol and Ethanoic acid (only properties
and uses),
soaps and detergents.
Periodic classification of elements: Need for classification, early attempts
at
classification of elements (Dobereiner’s
Triads, Newland’s
Law
of
Octaves,
Mendeleev’s Periodic Table), Modern periodic table, gradation in properties, valency,
atomic
number, metallic and non-metallic properties.
Theme: The World of the Living
(50 Periods) Unit
II: World of Living
Life processes: ‘Living Being’. Basic concept of nutrition, respiration, transport and
excretion in plants and animals.
Control and co-ordination
in
animals
and plants:
Tropic movements in
plants; Introduction of plant hormones; Control and co-ordination in animals: Nervous system;
Voluntary, involuntary and reflex action; Chemical co-ordination: animal hormones.
Reproduction: Reproduction in animals and plants (asexual and sexual) reproductive
health-need and methods of family planning. Safe sex vs HIV/AIDS. Child bearing and
women’s
health.
Heredity and Evolution: Heredity; Mendel’s contribution- Laws for inheritance of traits: Sex
determination: brief introduction; Basic
concepts of evolution.
Theme : Natural Phenomena
(23 Periods) Unit
III: Natural Phenomena
Reflection of light by curved surfaces; Images formed by spherical mirrors, centre of
curvature, principal axis, principal focus, focal length, mirror formula (Derivation not
required), magnification.
Refraction; Laws
of
refraction, refractive index.
Refraction of light by spherical lens; Image formed by spherical lenses; Lens formula
(Derivation not required); Magnification. Power
of
a lens.
Functioning of a lens in human eye, defects of vision and their corrections, applications of spherical mirrors
and lenses.
Refraction of light through a prism, dispersion of light, scattering of light, applications in
daily life.
Theme: How Things Work
(32 Periods) Unit
IV: Effects of Current
Electric current, potential difference and electric current. Ohm’s
law; Resistance,
Resistivity, Factors on which the resistance of a conductor depends. Series combination
of
resistors, parallel combination of resistors and its applications in daily
life. Heating
effect of electric current and its applications in daily life. Electric power, Interrelation between P, V, I and R.
Magnetic effects of current : Magnetic field, field lines, field due
to a current carrying
conductor, field
due
to current carrying coil or
solenoid; Force on current carrying conductor,
Fleming’s
Left
Hand Rule,
Electric Motor, Electromagnetic
induction. Induced potential difference, Induced current. Fleming’s Right Hand Rule, Electric Generator, Direct current. Alternating current:
frequency
of
AC. Advantage of AC over DC. Domestic electric circuits.
Theme: Natural Resources
(20 Periods) Unit
V: Natural Resources
Sources of energy:
Different forms of
energy, conventional and non-conventional sources of energy: Fossil
fuels, solar energy; biogas; wind, water and
tidal energy; Nuclear energy. Renewable versus
non-renewable sources of Energy.
Our environment: Eco-system, Environmental
problems, Ozone depletion, waste
production and their solutions. Biodegradable and non-biodegradable substances. Management
of
natural
resources: Conservation
and
judicious use
of natural resources. Forest and wild life; Coal and Petroleum conservation. Examples of people’s
participation for conservation
of natural
resources. Big
dams:
advantages and limitations; alternatives, if any. Water
harvesting. Sustainability of natural resources.
PRACTICALS
Practical should be
conducted alongside the concepts taught
in theory classes
LIST OF EXPERIMENTS
1. A. Finding the pH of the following samples
by
using pH paper/universal indicator:
(i) Dilute Hydrochloric Acid
(ii) Dilute NaOH solution
(iii) Dilute Ethanoic
Acid solution
(iv) Lemon juice
(v) Water
(vi) Dilute Hydrogen Carbonate solution
B. Studying the properties
of
acids and bases
(HCl
& NaOH) on the basis of their
reaction with:
a) Litmus solution (Blue/Red)
b) Zinc metal
c) Solid sodium carbonate
2. Performing and observing the following reactions and classifying them into:
A. Combination reaction
B. Decomposition reaction
C. Displacement reaction
D. Double displacement reaction
(i) Action of water on quicklime
(ii) Action of heat on ferrous sulphate crystals
(iii) Iron nails kept in copper
sulphate solution
(iv) Reaction between sodium sulphate and barium chloride solutions
3. Observing the action of Zn, Fe, Cu and Al
metals
on
the following salt solutions:
i) ZnSO4(aq) ii) FeSO4(aq) iii) CuSO4(aq)
iv) Al2 (SO4)3(aq)
Arranging Zn, Fe, Cu and
Al
(metals) in the
decreasing
order of reactivity based on the
above result.
4. Studying the dependence of potential difference (V)
across a resistor on the current (I)
passing through it and determine its resistance. Also plotting a graph between V and I.
5. Determination
of
the equivalent resistance of two resistors when connected
in
series and
parallel.
6. Preparing a temporary
mount of a leaf peel to show stomata.
7 Experimentally show that carbon dioxide is
given out during respiration.
8 Study of the following properties
of
acetic acid (ethanoic acid):
i) odour
ii)
solubility in water
iii) effect on litmus
iv) reaction with Sodium Hydrogen Carbonate
9 Study of the comparative cleaning capacity of a sample of soap in soft and hard
water.
10 Determination of the focal length of:
i) Concave
mirror
ii)
Convex lens
by obtaining the image of a distant object.
11 Tracing the path of a ray of light passing through a rectangular glass slab for different angles of incidence. Measure
the angle
of
incidence, angle of refraction, angle of
emergence and interpret the result.
12 Studying (a) binary fission in Amoeba, and (b) budding in yeast and Hydra with the help of prepared
slides.
13 Tracing the path of the rays
of
light through a glass prism.
14 Finding the image distance for varying object distances
in
case of a convex lens and drawing corresponding ray diagrams
to
show the nature of image formed.
15 Identification
of
the different parts of an embryo of a dicot seed
(Pea, gram or red kidney bean).
PRESCRIBED BOOKS:
Science-Textbook
for class IX-NCERT Publication
Science-Text book for class X- NCERT Publication
Laboratory Manual-Science-Class IX, NCERT Publication
Laboratory Manual-Science-Class X, NCERT Publication
Exemplar
Problems Class IX – NCERT Publication
Exemplar
Problems Class X – NCERT
Publication
1) Board
Examination –Theory
QUESTION PAPER DESIGN Class: IX AND X
(2019-20)
Subject: Science
(086)
Maximum Marks: 80
Duration : 3 Hours
|
Sr.
|
Typology of Questions
|
Objective
|
SA
|
LA
|
Total
|
|
No.
|
Type
*
|
(03 marks)
|
(05 marks)
|
||
|
|
(01 mark)
|
|
|
||
|
1
|
Remembering: Exhibit
memory
of
|
07
|
02
|
01
|
22.5%
|
|
previously learned
material by recalling
facts, terms, basic concepts, and
answers.
|
|||||
|
2
|
Understanding: Demonstrate
|
04
|
02
|
02
|
25%
|
|
understanding of facts and ideas
by
organizing,
comparing, translating, interpreting, giving descriptions, and stating
main
ideas
|
|||||
|
3
|
Applying: Solve problems
to new situations
by applying acquired
knowledge,
facts,
techniques and rules in a different way.
|
04
|
01
|
02
|
21.25%
|
|
4
|
Analyzing and Evaluating: Examine and
break
information into parts by
identifying motives or causes. Make inferences
and find evidence
to support generalizations
Present and defend opinions by
making
judgments about information, validity
of
ideas, or quality
of work based on a
set of criteria.
|
05
|
02
|
01
|
20%
|
|
5
|
Creating: Compile information together in
a different way by combining elements in a
|
-
|
03
|
-
|
11.25%
|
|
new pattern
or
proposing
alternative
|
|||||
|
solutions.
|
|||||
|
|
Total
|
20 (20)
|
10 (30)
|
06 (30)
|
100%
|
All questions would
be compulsory. However, an internal choice of approximately 33% would
be provided.
2) Internal Assessment: 20 Marks
Periodic Assessment – 05 marks + 05
marks
Subject Enrichment (Practical Work) – 05
marks
Portfolio – 05
marks
Note: Objective Section would have 10 MCQ. Besides this, the section would include VSA, Assertion-Reasoning type
questions
etc.